Clinical Research

The main clinical focus is on developing new therapies for rare tumors and vascular anomalies. Because present drug development requires rigorous testing involving hundreds to thousands of patients for each specific tumor type, patients with rare tumor types or unusual vascular lesions are infrequently studied in the setting of clinical trials. Rational drug development in this group of patients therefore involves individualized treatment plans that are based on careful identification of proteins overexpressed in the pathological lesion. This approach is also gaining in importance in difficult to treat, multiply–recurrent cancers such as sarcomas or brain tumors. Following are examples of targeted therapies for rare tumors:
Interferon for Juvenile Nasopharyngeal Angiofibroma (JNA)
Juvenile nasopharyngeal angiofibroma (JNA) is a well-known clinical entity. It was described as far back as fifth century BC by Hippocrates. JNA accounts for 0.05% of all head and neck tumors, with frequency varying from 1:5,000 to 1:60,000 of all otolaryngology patients. It typically presents with chronic nasal congestions and nose bleeds in adolescent males. the treatment of choice is surgery, but in some situations this may not be possible due to the size, location and hemorrhagic nature of these tumors.The tumor, even though benign is locally invasive, aggressive, and frequently re-grows (2).
It is unclear what causes JNA, and this lack of understanding has hindered the development of novel therapies. A number of theories exist. For one, the tumor growth may be the result of somatic genetic mutation in the nonchromaffin paraganglionic cells of the terminal branches of the maxillary artery (3,4). Alternatively, the aggressive growth may be due to, or be exacerbated by, the anabolic effect of pubertal hormones on the vasculature (5-7). Finally, the neoplasm may represent a desmoplastic response (8).
In contrast to the lack of understanding about its causes, the clinical course is well known and consistent. The lesion originates in close proximity to the posterior attachment of the middle turbinate, and grows anteriorly as well as posteriorly. The resulting tumors are frequently bilobed or dumbbell-shaped, with one portion of the tumor filling the nasopharynx and the other portion extending to the pterygopalatine fossa. In many cases tumor progresses beyond the nasal cavity, extends superiorly and erodes into the brain. In some of the most severe cases, the tumor invasion of orbital fissures, leads to proptosis and optic nerve atrophy. For those very difficult cases, where only partial resection is possible, no good non-surgical alternatives exist.
Because the aggressive progression of this very vascular tumor involves active angiogenesis (the growth of new vessels) (8-11) , a good rationale exists for adjuvant anti-angiogenic therapy with / or without antihormonal therapy (6,7).
Presently available therapeutic alternatives such as radiotherapy (12), stereotactic radiotherapy (ie, Gamma knife), three-dimensional conformal radiotherapy/ surgery (13), or embolization, have had variable degree of success. No single modality has had consistent results. Endovascular therapy (embolization), while very useful in acute management, often results in rapid re-growth. Radiation, even though quite effective, represents a less desirable modality in most cases because of concerns about long-term effects on bone growth and secondary malignancies.
Traditional chemotherapy has rarely been explored for JNA, as the toxicity and the long term sequelae of traditional cytotoxic agents could not be justified in this otherwise benign tumor. However, the recent emergence in oncology of biologic modifiers, and in particular anti-angiogenic agents, has for the first time provided a non-toxic alternative for this highly angiogenesis-dependent tumor. We reviewed seven cases of JNA that presented to ORL at Children’s Hospital from 2003-2008, in whom all of the available therapeutic options such as radiation or surgery would have been associated with significant risks or long term effects, and who were treated with experimental antiangiogenic therapy. They were treated with low dose, daily Interferon alpha 2B (Fidler ref), with the goal to reduce the tumor bulk and make surgical resection with/ or without perioperative endovascular therapy a safer alternative. All of the treated cases represented the most severe situations; with multiply recurrent or progressive JNA.
Interferon alpha 2B is an anti-angiogenic agent with an excellent therapeutic profile and no known long-term toxicity in this age group. In infants its use can be associated with poor myelination and secondary spastic dipledgia (Kaban Ref), but this risk decreases with advancing age, and is very low by puberty, which is when most cases of JNA occur. The acute side effects such as flu-like symptoms, transaminitis and leucopenia are much reduced by the low dose, continuous regimen used in this study. We report that therapy with low-dose, daily Interferon alpha 2B resulted in a significant decrease in tumor size and vascularity in all of the treated cases, allowing for a safer and more efficacious surgical management. Furthermore, in cases where significant residual remained following resection IFN alpha 2B prevented re-growth in all of the treated cases.
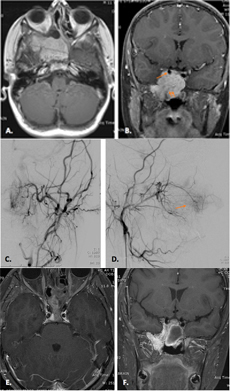
Consecutive cases of JNA were evaluated in a multidisciplinary conference that included representatives from ORL, radiation oncology, interventional radiology and oncology. Only patients with disease not amenable to safe surgical or endovascular management were given the choice of experimental anti-angiogenic therapy. The complications included: multiply recurrent disease in one patient (Figure 1), severe proptosis and or intracranial extension (Figure 2), clinical signs referable to intracranial extension of the tumor (three out of the seven patients, Figure 3), and severe intractable epistaxis (two out of the seven patients).
All of the identified children (seven to date) were consented to an individualized treatment protocol, as per institutional guidelines. CT scan and/or MRI used to demonstrate lesion origin, extent, and enhancement pattern, were performed at baseline, and as indicated by clinical situation. All data has been collected and summarized following an approval of an IRB approved retrospective review.
